
Recently, the experimental team led by Researcher Jiang Ling and Associate Researcher Xie Hua from the Cluster Spectroscopy and Dynamics Research Group (Group 2506) of the State Key Laboratory of Molecular Reaction Dynamics at Dalian Institute of Chemical Physics, in collaboration with the theoretical team led by Associate Professor Hu Hanshi from Tsinghua University, employed an independently developed infrared-extreme ultraviolet (IR-VUV) infrared spectroscopy experimental device. In the reactions of IIIB group transition metals (Sc, Y, La) with CO, the hept-coordinated metal carbonyl compound Sc(CO)7 and the octa-coordinated metal carbonyl compound TM(CO)8 (TM=Y, La) in the infinite domain of the gas phase were discovered, providing new ideas for the design of compounds with unique properties and precise regulation.
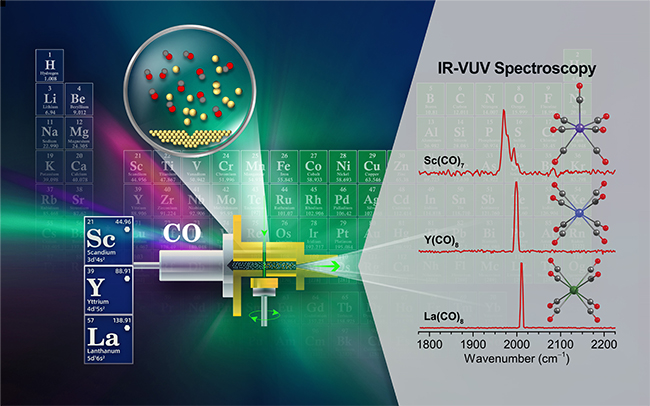
Metal carbonyl compounds not only provide a model system for studying metal-ligand bonding and chemical rules, but also play an important role in many catalytic processes, such as Fischer-Tropsch, hydroformylation and alcohol synthesis. The preparation and characterization of highly coordinated metal carbonyl compounds is one of the most challenging scientific problems. Compared with ionic metal carbonyl compounds, neutral metal carbonyl compounds are difficult to detect and select in terms of mass due to the lack of charge, making experimental research very challenging. Therefore, achieving the preparation and spectral characterization of neutral highly coordinated metal carbonyl compounds has long been one of the goals of scientists.
In recent years, in order to achieve precise detection and structural analysis of neutral metal carbonyl compounds, Jiang Ling's team has made significant progress in related experimental techniques. They independently developed high-throughput laser sputtering cluster sources and established an infrared - extreme ultraviolet (IR-VUV) infrared spectroscopy experimental method based on Dalian Coherent Light Source (Rev. Sci. Instrum. In 2020, and by using this method, carbon-carbon coupling reactions were discovered in neutral titanium carbonyl compounds (J. Phys. Chem. Lett., 2021), which interpreted the insertion reaction mechanism of metals to carbon monoxide from a brand-new perspective.
In this work, the team prepared neutral Sc(CO)7 and TM(CO)8 (TM=Y, La) products by using the independently developed neutral cluster infrared spectroscopy experimental device. The infrared spectra of infrared lasers were determined by combining infrared laser vibration pre-dissociation with 193nm extreme ultraviolet laser ionization generated by excimer lasers. The stable configurations and infrared spectra of these products obtained by Hu Hanshi's team through high-precision quantum chemistry theoretical calculations are highly consistent with the experimental spectra. Studies show that Sc(CO)7 has a C2v structure, and TM(CO)8 (TM=Y, La) has a D4h structure. The calculated thermodynamic data indicate that their formation is a thermodynamically exothermic and kinetically feasible process under gas-phase conditions. Bonding analysis indicates that among these products, the TM→CO feedback π bond causes the C-O stretching vibration frequency to redshift relative to CO, and the feedback π bond effect weakens with the increase of the atomic number of the central atom. When only the valence electrons occupying the TM-CO bond orbitals are considered, these high-coordination carbonyl compounds all have 17 valence electrons. This work discovered transition metal carbonyl compounds with seven-coordination and eight-coordination in the infinite domain of the gas phase, which is conducive to promoting the development of size-resolved neutral cluster infrared spectroscopy and provides a new strategy for designing, preparing and characterizing various compounds with unique properties at the atomic and molecular levels.
The relevant research results are based on "Observation of Confinement-Free Neutral Group Three Transition Metal Carbonyls Sc(CO)7and TM(CO)8(TM = Y) The title "La)" was recently published in Angewandte Chemie International Edition. The co-first authors of this work are Wang Chong, a doctoral student from Group 2506 of Dalian Institute of Chemical Physics, and Tian Changyi, a doctoral student from Tsinghua University. This work was supported by the Major Project of Science and Technology Innovation 2030 of the Ministry of Science and Technology, the Science Center Project of "Frontier Research on Dynamic Chemistry" of the National Natural Science Foundation of China, the Special Fund of Dalian Coherent Light Source, and other projects. (Written and photographed by Wang Chong and Tian Changyi
The article links: https://doi.org/10.1002/anie.202305490
(The text and images are reprinted from the homepage of Dalian Institute of Chemical Physics, Chinese Academy of Sciences)