
Recently, a research team led by Professor Wang Zhandong from the National Synchrotron Radiation Laboratory and the State Key Laboratory of Fire Safety at the University of Science and Technology of China (USTC) has achieved a significant advance in understanding the nucleation mechanism of black carbon (soot) aerosols. By combining experimental and theoretical approaches, the team discovered that covalent cluster intermediates generated during combustion act as critical bridges in the gas-to-particle transition of small molecules to soot particles. They proposed a novel nucleation mechanism termed "Resonance-Stabilized Radical Clustering (RSRC)". The findings, titled "Resonance-stabilized radical clustering bridges the gap between gaseous precursors and soot in the inception stage", were published online in the Proceedings of the National Academy of Sciences (PNAS).
Black carbon aerosols, produced by incomplete combustion of fossil fuels and biomass, are key components of atmospheric fine particulate matter (PM2.5) and exert multidimensional environmental impacts: they degrade regional air quality, exacerbate respiratory diseases, and absorb solar radiation, altering atmospheric energy balance and ranking as the second-largest contributor to global warming after carbon dioxide. In energy systems, soot deposition (engine coking) reduces combustion efficiency and accelerates mechanical wear. Despite established knowledge of their environmental risks, the core scientific question of their nucleation mechanism—specifically, the dynamic pathway from gaseous precursors to particulate phases and the molecular interface reactions during critical nucleation stages—remained unresolved. This knowledge gap has hindered advancements in pollution control technologies (e.g., clean combustion engines), environmental policy optimization, and interdisciplinary modeling of atmospheric chemistry, cloud physics, and climate systems.
Building on their prior discovery of persistent free radical reactions generating covalent cluster intermediates and soot particles (JACS, 2024, 146, 19, 13571–13579), the team designed pyrolysis experiments using 1-indenyl, 1-methylnaphthyl, and typical transportation fuel components as reactants to synthesize nascent soot particles. Laser desorption/ionization mass spectrometry revealed the presence of covalent clusters within the particles (Figure 1). Further analyses—including rotary evaporation/vaporization, synchrotron radiation photoionization mass spectrometry, thermogravimetry, and cluster molecular dynamics simulations—demonstrated that gas-phase small-molecule clusters exhibit distinct particulate-phase growth characteristics upon reaching a critical mass, with phase transition sizes (1.3–1.6 nm) closely matching experimentally observed nascent soot particle dimensions (Figure 2).
The proposed RSRC mechanism highlights covalent cluster formation via radical chain reactions as essential to soot nucleation, contrasting sharply with traditional theories that attribute soot nucleation to physical stacking or weak chemical associations of polycyclic aromatic hydrocarbons. This breakthrough provides molecular-level insights into the initial nucleation process and establishes a theoretical foundation for multiscale modeling of soot aerosol dynamics.
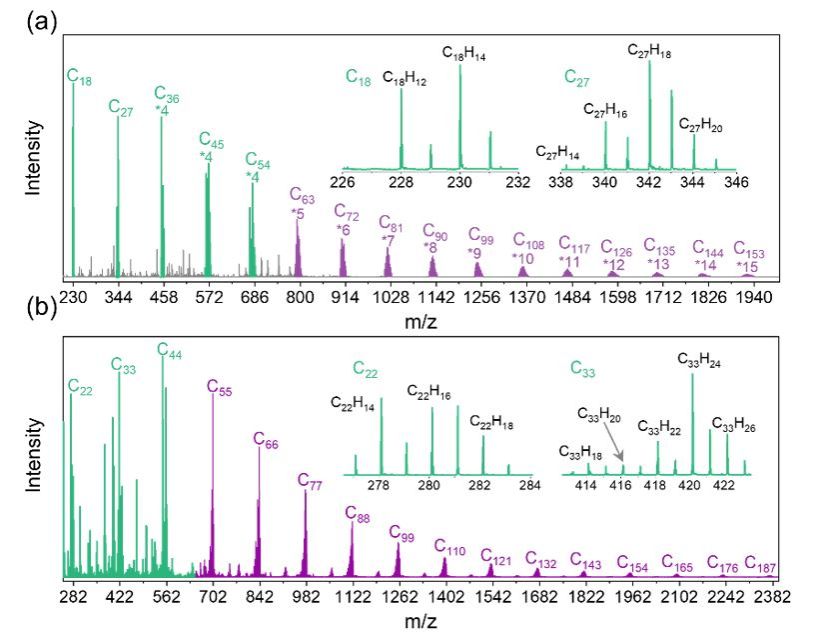
Figure 1. Covalent clusters of varying molecular weights detected in soot aerosols.
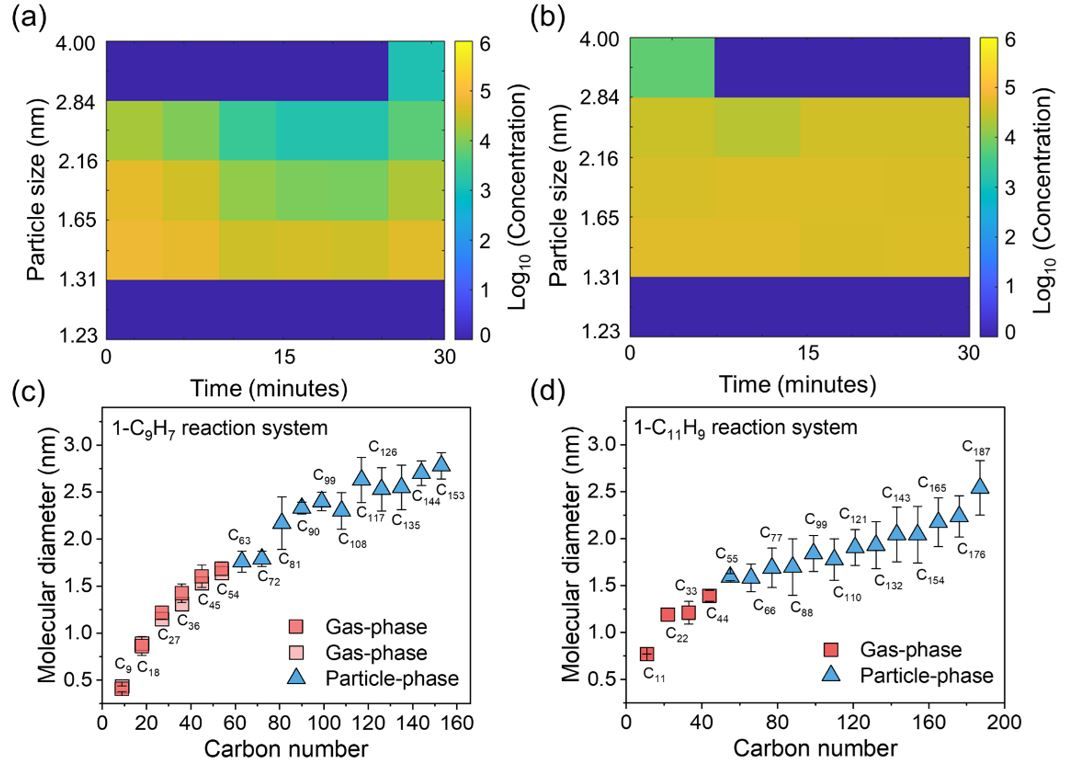
Figure 2. (a–b) Nascent soot particles (1.3–1.6 nm) observed in 1-indenyl and 1-methylnaphthyl pyrolysis experiments. (c–d) Theoretical simulations predict phase transition sizes of ~1.5 nm for covalent clusters, aligning closely with experimental data.
Ph.D. candidate Wang Hong from USTC’s National Synchrotron Radiation Laboratory is the co-first author, with Guan Jiwen and Wang Zhandong serving as corresponding authors. The study was supported by the National Key Research and Development Program of China Young Scientists Project (2021YFA1601800) and the National Natural Science Foundation of China (62310000379).
Paper Link:https://doi.org/10.1073/pnas.2503292122