
Recently, the team led by Researcher Jiang Ling and Associate Researcher Xie Hua from the Cluster Spectroscopy and Dynamics Research Group (Group 2506) of Dalian Light Source Research Laboratory, Dalian Institute of Chemical Physics, utilized photoelectron spectroscopy experimental methods to study the reaction mechanism between the heterodinuclear metal cluster FeV - and nitrogen, revealing that FeV - can break the N≡N triple bond at room temperature. It provides new ideas for the research and development of inorganic and biological nitrogen fixation.
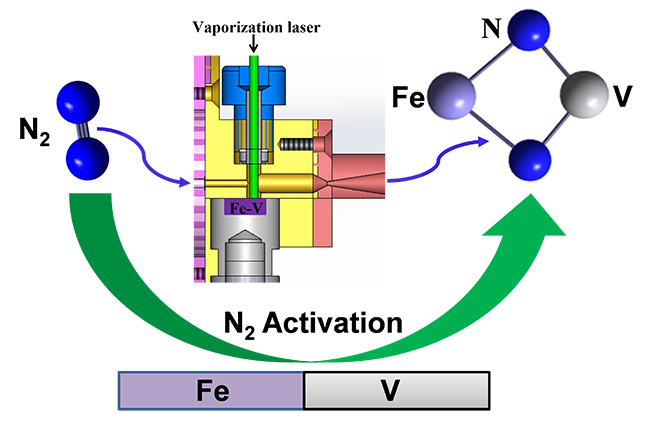
Nitrogen, as the most abundant resource in the atmosphere, converting it into nitrogen-containing compounds is of great significance to human production and life. The bond energy of the N≡N triple bond of nitrogen gas is extremely high (approximately 226kcal/mol), and it is difficult to activate or break. Currently, in industry, the traditional high-temperature and high-pressure Haber-Bosch process is still mainly used for nitrogen fixation. Therefore, achieving efficient nitrogen fixation under mild conditions has long been the dream of scientists.
The research team led by Jiang Ling and Xie Hua has been dedicated to the study of the reaction mechanism between metal clusters and N2. In the previous related research (Angew. Chem. Int. Ed., 2017; J. Phys. Chem. Lett., 2022; Based on Inorg. Chem., 2023, the team generated FEVN2-negative ions by laser sputtering cluster sources and obtained their rich electronic structure information. Combined with quantitative calculations, they determined their stable four-membered ring structure. Studies have shown that FeV - can effectively activate and decompose N2 at room temperature, and its natural charge achieves the transfer from FeV - to N2. This result reveals the reaction mechanism of heteronuclear bimetallic atoms synergically activating N2, providing a new strategy for efficient nitrogen fixation.
the related results were published in JACS Au under the title "Dinitrogen Activation by Heteronuclear Bimetallic Cluster Anion FeV-in the Gas Phase". The first author of this work is Du Shihu, a jointly trained postgraduate student from Group 2506 of Dalian Institute of Chemical Physics. The above research work was supported by the Major Project of Science and Technology Innovation 2030 of the Ministry of Science and Technology, the Science Center Project of "Frontiers in Dynamic Chemistry" of the National Natural Science Foundation of China, the Youth Innovation Promotion Association of the Chinese Academy of Sciences, and the Special Fund of Dalian Coherent Light Source, among others. (Written and photographed by Du Shihu
The article links: https://pubs.acs.org/doi/10.1021/jacsau.3c00143