- Facilities at a Glance
- All Facilities
- Material
- Earth System and Environment
- Engineering Technology
- Space and Astronomy
- Particle and Nuclear Physics
- Energy
- Biology
- 1
- 2
- 3
National Facility for Protein Science in Shanghai (NFPS)
Create Proposal The National Facility for Protein Science Shanghai (NFPS)project was approved by National Development and Reform Committee (NDRC) in 2008 with its feasibility study report signed off by NDRC in later 2010. NFPS, officially opened to users from different institutes, universities, hospitals and companies since 28th July, 2015, welcome users from all over the world to carry out experiments and pursue groundbreaking discoveries in protein science.
The facility is composed of 9 technology systems with state-of-the-art instruments in its Haike Road Campus and 6 Beamline stations within the Shanghai Synchrotron Radiation Facility. These sysytems are Large-scale Pritein Prodction, Protein Crystal Structure Analysis, Nuclear Magnetic Resonance, Electron Microscopy, Protein Dynamic Analysis, Mass Spectrometry, Integrated Laser Microscopy, Molecular Imaging and Data Base & Computation.
A group of talented technical staff will be in place managing the facility to ensure the facility staying at high efficiency and competency in the field.
If you would like to know more about 9 technology systems, please refer to Instruments List.
Equipment
-

Electron Microscopy System
电镜分析系统主要运用低温冷冻透射电镜,探索三维重构技术的发展与应用,以期达到对生物超大分子复合体的高分辨率三维结构解析。目前,该技术主要应用为单颗粒三维重构及电子断层三维成像。电镜分析系统现配有三台FEI低温冷冻透射电子显微镜,包括:300千伏场发射Titan Krios(配备K2直接电子探测器)、200千伏场发射TF20(配备Falcon II探测器),以及120千伏LaB6光源的T12。提供不同加速电压下的冷冻电镜观察服务,以单颗粒重构和三维断层扫描重构等实验方法为主,建立起生物大分子复合体、膜蛋白复合体、蛋白质分子机器、病毒(分子量为100 kDa以上)等,以及细胞、细胞器等大尺度三维结构的解析能力。样品制备模块可满足快速冷冻制样、负染样品制备、高压冷冻、超薄切片、免疫和组织细胞切片分析等制样要求。
-

Electron Microscopy System-Tecnai G2 Spirit
The FEI Spirit provides high-contrast and high-resolution images of plastic-embedded thin-sectioned material, negative stained samples, Cryo-EM single particle and tomography.
-

Electron Microscopy System-Tecnai TF20
The TF20 is a 200kV FEG TEM, it has a Twin objective Lens, is equipped with Falcon II direct detection camera and is dedicated to only Cryo-EM applications. Cryo-EM data can be collected manually, or automaticaly using Serial EM or EPU.
-

Electron Microscopy System-Titan Krios
300 kV FEG Titan Krios has a C-Twin objective Lens, it is equipped with Cs corrector, K2 Summit direct detection camera and Gatan GIF energy filter.Cryo-EM data can be collected manually, or automaticaly using Serial EM.
-

Nuclear Magnetic Resonance System
T
1)Study protein structure, dynamics and conformation exchange procedure in solution.
2)Study protein-protein and protein-ligand interaction, especially weak interaction.
3) Study membrane protein structure and dynamics.
4) Study intrinsically disordered protein and RNA conformation feature.
5) Bio-NMR technology and methodology study.
-

Nuclear Magnetic System-Agilent 600 MHz DD2 spectrometer
14.1 Tesla, 54 mm bore PremiumCOMPACT superconducting magnet. The Direct Drive 2 (DD2) console features DirectDigital Receiver(DDR), new transmitter boards and phase shifters which provide superior performance. The 600 MHz spectrometer with 5 mm PFG Triple Resonance 13C Enhanced cryoprobe is used for biomolecular applications, fragment-based screening, metabolomics and small molecule NMR.
-

Nuclear Magnetic System-Agilent 700 MHz DD2 spectrometer
16.44 Tesla, 54 mm bore Premium superconducting magnet. The Direct Drive 2 (DD2) console features DirectDigital Receiver(DDR), four radio frequency (RF) channels with waveform generators and XYZ pulse-field gradient module. The 700 MHz spectrometer, equipped with a 5 mm triple-axis pulsed field gradient triple resonance RT probe and a 3.2 mm BioMAS solid probe, is capable of high-resolution liquid- and solid-state NMR.
-

Nuclear Magnetic System-Agilent 800 MHz DD2 spectrometer
18.84 Tesla, 54 mm bore PremiumCOMPACT superconducting magnet. The Direct Drive 2 (DD2) console features DirectDigital Receiver(DDR), new transmitter boards and phase shifters which provide superior performance. The 800 MHz spectrometer with 5 mm PFG Triple Resonance 13C Enhanced cryoprobe is used for biomolecular applications, fragment-based screening and small molecule NMR requiring high sensitivity.
-

Nuclear Magnetic System-Bruker 600 MHz Avance IIITM spectrometer
14.1 Tesla, 54 mm bore magnet ,ultraStabilized and ultraShield. The console features four radio frequency (RF) channels for observe, pulse, and decoupling functions and the 5 mm Triple Resonance Inverse (TCI) cryoprobe with Z-Gradients. The 600 MHz spectrometer ,equipped with an automated sample changer (24 samples) and a Nitrogen Liquefier, is used for biomolecular applications, fragment-based screening, metabolomics and small molecule NMR.
-

Nuclear Magnetic System-Bruker 900 MHz Avance IIITM spectrometer
21.14 Tesla, 54 mm bore US2 magnet which combines Bruker’s high performance UltraShieldTM active-shielding and UltraStabilizedTM sub-cooling technologies. The console is equipped with 4 radio frequency (RF) channels for observe, pulse, and decoupling functions and the 5 mm Triple Resonance Inverse (TCI) cryoprobe with Z-Gradients . The 900 MHz spectrometer provides cutting-edge research capability that is critical for a number of investigations on biomolecular system that play a significant role in disease.
-

规模化蛋白质制备系统
规模化蛋白质制备系统自主设计搭建了五套大型自动化设备,将软件控制,硬件设备和生物应用结合在一起,实现了高通量蛋白质表达条件的自动化筛选。整个流程从大规模PCR扩增开始,高通量重组质粒的构建,细胞生长,诱导表达,蛋白纯化(构建了大肠杆菌,昆虫细胞,哺乳动物细胞三种表达体系),最终实现对蛋白质表达条件全方位多途径的优化,完全取代了在传统实验室中的单线重复手工操作,达到全球生物自动化一流水平,极大的提高了生物实验效率。
-

质谱分析系统
主要通过对蛋白质的高分辨、高准确性的质谱鉴定,大规模地确定功能系统中起重要相互作用的蛋白质化合物,从而提示进行结构分析和分子影像分析发现蛋白质的功能;通过质谱分析定位发生在蛋白质上的修饰位点,进一步指导蛋白质结构的测定和功能分析;此外,通过对重要功能蛋白质的精确定量分析追踪在不同时间和处理条件下的蛋白质结构变化,从而为解释细胞活动的分子机制,筛选疾病生物标志物和药物靶 点提供分子基础。具体可提供服务:蛋白质分子量测定,蛋白质鉴定,差异蛋白质组分析,蛋白质后修饰分析,定量蛋白质组学分析等。
-

复合激光显微成像系统
复合激光显微成像系统包括荧光动态成像、细胞和组织水平成像以及高通量细胞成像分析分选设备。主要用于研究蛋白质细胞定位、组织或细胞的微细结构和蛋白质分子在细胞内的动态过程等,在观察活组织内蛋白质的结构和功能的动态变化、以及细胞网络的协同活动方面有突出优势。复合激光显微镜系统由转盘式激光共聚焦显微镜、超高分辨率显微镜、双光子显微成像、高通量细胞分析四个功能模块构成。
-

Beamlines-BL01B- Infrared Beamline
(1)Infrared microspectroscopy and imaging experimental station
Instrument: Nicolet 6700 FTIR Spectrometer coupled to a Continuum Microscope
Spectral range: 600-10000 cm-1
Spectral resolution: 0.1cm-1
Flux at the sample: > 1013 (photons/sec/0.1% bw) @ 1μm @300mA
Focused beam size: 18μm@1000cm-1 (diffraction limit spatial resolution)
Minimum aperture dimension: 5×5μm
(2)Time-resolved infrared spectroscopy experimental station
Instrument: Nicolet 8700 FTIR Spectrometer
Spectral range: 10-10000 cm-1
Spectral resolution: 0.1 cm-1
Flux at the sample: > 1013 (photons/sec/0.1% bw) @ 1μm @300 mA
Time resolution: 10 ns with step-scan mode -

Beamlines-BL17B-High-throughput Crystallography Beamline
Nowadays macromolecular crystallography has become the dominant technique for protein structural determination. As the crystallization method developed, scientists get the crystals from proteins more quickly than before. The demand for beam time is rapidly increasing. High-throughput crystallography beamline is dedicated for high efficient and automatic data collection, thus it will not pursue the difficult cases of structural determination but will be very efficient for the crystal screening and structural determination in a large amount, to serve for a large number of users for conventional experiments in a cost-effective way. This beamline is designed to be a tunable beamline which can be used to carry out multi-wavelength anomalous diffraction (MAD) experiment. The energy range is from 5~20keV, which covers most of the absorption edges of the common-used anomalous elements. This beamline is composed of the bending magnet source, the front end, the beamline, and the experimental station. The energy range is from 5~20 keV, the energy resolution is less than 3×10e-4 (@12keV), the Flux at sample is larger than 5×10e11 ph/s (12keV @300mA), and focused beam size is within 120×80 μm2 (H×V, 12keV), and the focused beam divergence is within 1.5×0.2 mrad2 (H×V, @12keV).
This beamline is an advanced data collection facility at NFPS/SSRF to the national user community and maintains high-profile user programs. It exploits major advances in x-ray crystallography and not only addresses the structural biology problems, but also addresses studies on material and chemical crystal structure. The MarCCD MX325 detector is equipped at this beamline. MD2 Microdiffractometer with high-speed and high-precision air bearing goniometer is used as the sample holder and sample centering system. It also equips with an automated sample changer, which makes it possible to collect data remotely. -

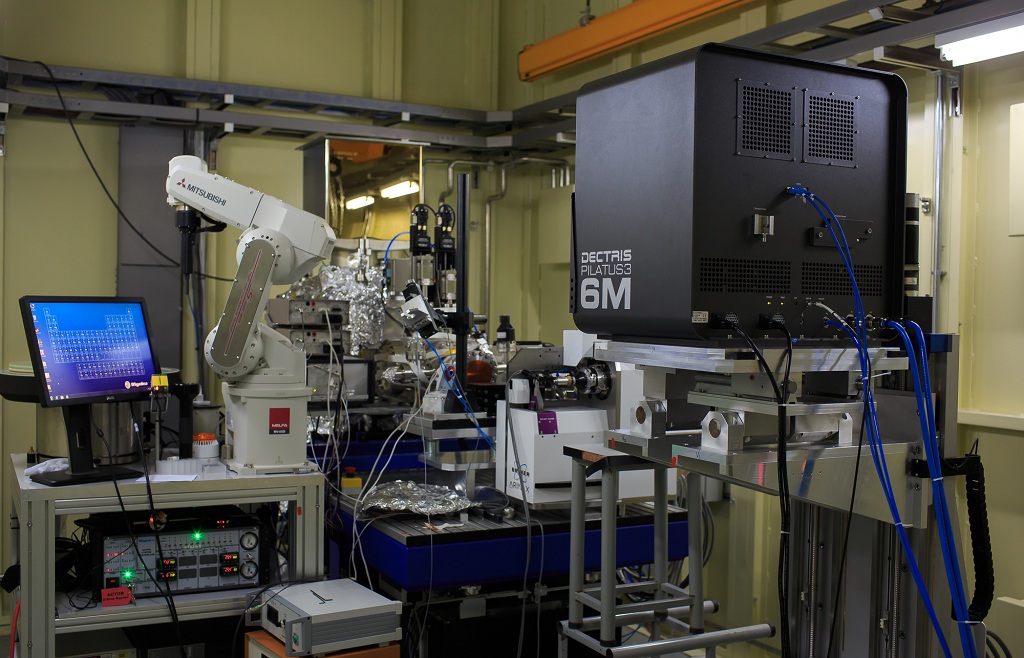
Beamlines-BL18U-Protein Micro-Crystallography Beamline
Protein crystal growing is one of the most important processes in macromolecular crystallography technique, while it is a very complicated and difficult process too. Many proteins, especially the membrane proteins, have difficulties in growing single crystals of sufficient orderliness and size. If protein crystallography with micro-sized crystals could be achieved, the efficiency and success rate will be greatly improved.
Protein micro-crystallography beamline should be able to deal with the micro-crystals of size down to a few microns. This will make many formidable tasks of structural determination much easier. It is also the typical crystal size of lipid-cubic-phase crystallization method, developed for the membrane protein crystal growth. This beamline is designed to provide stable and highly brilliant micro-beam. It is expected and requested to be a powerful tool to obtain diffraction data from protein micro-crystals. The light source of this beamline can easily cover the energy range of 5-18 keV and can meet the requirement of the SAD techniques based on S and Se element. It has also covered the absorption edged of most important elements, such as Cr, M, K, Xe, Cs, Ba.
This beamline is composed of the undulator source, the front end, the beamline, and the experimental station. The energy range is from 5~18 keV, the energy resolution is less than 2×10e-4 (@12keV), the Flux at sample is larger than 5×10e11 ph/s (12keV @300mA), the focused beam size is within 10×5 μm2 (H×V, @12keV), and the focused beam divergence is within 0.7×0.25 mrad2 (H×V, @12keV).
This beamline is an advanced data collection facility at NFPS/SSRF to the national user community and maintains high-profile user programs. It exploits major advances in macromolecular x-ray crystallography and addresses the most challenging structural biology problems to advance scientific knowledge. The up-to-date detector Pilatus-3-6M is equipped at this beamline. MD2 Microdiffractometer with high-speed and high-precision air bearing goniometer is used as the sample holder and sample centering system. It also equips with an automated sample changer, which makes it possible to collect data remotely. Based on the dedicated equipment, shutterless data collection method is achieved, which make it possible to collect a set of data within 1 minutes. -

Beamlines-BL19U1-Protein Complex Crystallography Beamline
Structure determination of protein complexes is now the important direction of structural biology development. The structure of protein complexes and assemblies is the basis for interpreting the function of them. Its development goal is to study the increasingly complex protein complex system and the systematic function of molecular machines, organelles, such as virus, ribosome, etc. Protein complex crystallography beamline is aimed mainly at the structural determination of large protein complexes and assemblies, crystals of which typically have large unit cell and week diffraction, especially those of large protein complexes and membrane proteins with huge molecular weight and large unit-cell parameters (unit cell parameters up to 3000Å).
The brilliant and parallel beam is required to facilitate the structural determination of crystals of large unit cell. The big cell parameters in real space induce fine small reciprocal spacing, and the many diffraction points are difficult to separate from each other. In order to improve the resolution between individual points, the divergence of the incident X-Ray beam should be reduced: the smaller the divergence, the higher the resolution. The highly collimated x-ray will improve the diffraction data in following ways: it will shrink the broadening of the diffraction spot, thereby improving the signal–to-noise ratio, and this will have the effect to increase resolution of the crystal structure that is being determinated, especially for macromolecular complex crystals.
This beamline is composed of the undulator source, the front end, the beamline, and the experimental station. The energy range is from 7~15 keV, the energy resolution is less than 2×10e-4 (@12keV), the Flux at sample is larger than 1.5×10e12 ph/s (12keV @300mA), the focused beam size is within 120×80 μm2 (H×V, @12keV), and the focused beam divergence is within 0.1×0.1 mrad2 (H×V, @12keV).
This beamline is an advanced data collection facility at NFPS/SSRF to the national user community and maintains high-profile user programs. It exploits major advances in macromolecular x-ray crystallography and addresses the most challenging structural biology problems to advance scientific knowledge. The up-to-date detector Pilatus-3-6M is equipped at this beamline. MD2 Microdiffractometer with high-speed and high-precision air bearing goniometer is used as the sample holder and sample centering system. It also equips with an automated sample changer, which makes it possible to collect data remotely. Based on the dedicated equipment, shutterless data collection method is achieved, which make it possible to collect a set of data within 1 minutes. -

Beamlines-BL19U2- Biological Small Angle X-ray Scattering Beamline
As the first biological material dedicated SAXS beamline, the electrons of BL19U2 beamline come from undulator which could provide high brilliance for BL19U2 end-stations. A double flat silicon crystal (111) monochromator is used in BL19U2 with a tunable monochromatic photon energy ranging from 7 to 15 keV. The RMS source size is 380 μm (H) x 25 μm (V) and divergence 80 μrad (H) x 30 μrad (V). The focusing element of the beamline is composed of two mirrors. One is the horizontal focusing mirror situated at 31.2 m from the source. The other one is the vertical focusing mirror situated downstream at 34 m from the source. Typically, the beam is focused to 0.33 mm (H) x 0.05mm (V) in the detector plane (22 m from the second focusing mirror), being 0.45 mm (H) x 0.11 mm (V) at the sample position (19.85 m from the second focusing mirror). The beam size in the sample plane can be further reduced at the expense of the photon flux. SAXS data is collected using a Pilatus 1M detector with over one million pixels. A homemade automatic solution-sample-changing peristaltic device has been developed for standard BioSAXS measurements at BL19U2. Samples and buffers with a volume about 60ul are injected and undergo continuous flow during experiments to eliminate radiation damage induced by the intense X-ray beam.The efficiency of standard BioSAXS measurements is also improved by realization of automatic sample-changing and cleaning about this device. Inline HPLC-SAXS measurement mode is now available at BL19U2 for the analysis of polydisperse macromolecules which are difficult to analyze using standard BioSAXS measurement device. Biological samples elute from the analytical size exclusion columns (including Superdex 200 Increase 10/300, Superdex 75 Increase 10/300 columns from GE Healthcare and WTC-015S5,WTC-050S5 columns from Wyatt) which are attached to Agilent 1260 Infinity HPLC system, then go through Agilent UV and Wyatt MALS/DLS detectors(DAWN HELEOS II and DynaPro NanoStar) and finally flow into an in-beam quartz capillary for SAXS measurement. The HPLC-UV-MALS/DLS-SAXS mode can significantly improve the monodispersity of samples and provide information on possible oligomeric states. Commercial Linkam HFSX350-CAP heating/freezing stage (-196℃ to 350℃) is available for in situ BioSAXS analysis. Commercial Biologic SFM-2000 stopped-flow device is provided for users to perform time-resolved BioSAXS studies triggered by rapid solution mixing.Sufficient scattered photons can be collected in 30-100ms.Up to several millilitres of sample should be prepared due to the dead volume of the device and repeated measurements.
NOTICE
-
Call for Proposals for HEPS Phase II Beamlines May 23,2022