
Recently, a research team led by Researcher Yuan Kaijun and Academician Yang Xueming from Dalian Light Source Research Laboratory, in collaboration with Professor Xie Daiqian from Nanjing University, for the first time measured the hydrogen product channels in the photysis of water molecules and found that all these hydrogen products were in a vibration-excited state. This photochemical reaction provides an important way for the source of vibration-excited state hydrogen existing in interstellar space.
Hydrogen is the most abundant molecule in the universe and plays a very important role in the evolution of the universe. Interstellar observations have revealed a large amount of hydrogen gas in a vibrational excited state distributed in the nebula, especially in the interstellar light radiation region, more than 500 spectral lines from the vibrational excited state hydrogen gas have been observed astronomically. Vibration-excited hydrogen, due to its long lifetime and high reactivity, plays an important role in the composition and evolution of planetary atmospheres. Current interstellar theory indicates that the vibration-excited state of hydrogen mainly has two sources: the shock waves generated by stellar explosions or formation processes heat the hydrogen to the vibration-dynamic state, or the vibration-dynamic state where hydrogen is excited by ultraviolet light and then decays into the ground state of electrons. Theoretical predictions suggest that the direct formation of vibration-excited state hydrogen may also be an important source of these high-energy hydrogen gases, but the specific formation process remains unclear.
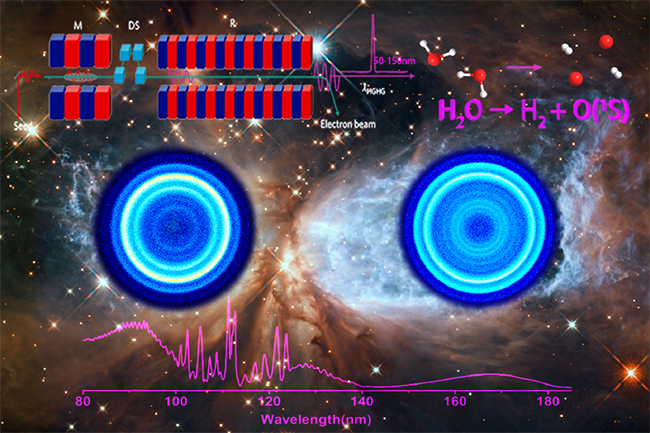
Using the Dalian Light Source, Yuan Kaijun's team systematically studied the photochemical process of water molecules. The dissociation wavelength was tuned to the range of 100 nanometers to 112 nanometers, and the O(1S)+H2 product channel was observed for the first time using ion imaging. Experiments show that hydrogen products are mainly distributed in the third or fourth vibrational excited state. Theoretical calculations have constructed the transition state structure of water molecules and explained the formation mechanism of hydrogen in the vibrational excited state. Given the widespread presence of water in the atmospheres of cosmic nebulae and comets, the photolysis of water molecules into the source of vibration-excited state hydrogen that exists in the interstellar light radiation region provides a new approach, which is of great significance for establishing models of the evolution of nebulae and planetary atmospheres.
This work represents a new breakthrough by Yuan Kaijun's team in systematically studying the extreme ultraviolet photochemical process of water molecules using the Dalian Light Source. Previous studies include the discovery of superheated hydroxyl radicals produced by the photolysis of water molecules (Nat. Comm., 2019), the observation of the formation of hydroxyl superrotors in electronically excited states (JPCL, 2020), and the accidental resonance effect induced by isotopes of water molecules (JPCL, 2019). The photolysis of water molecules to form highly vibration-excited OH is the source of Martian atmospheric glow (JPCL, 2020), and the three-body dissociation of water molecules to produce oxygen is an important source of oxygen in the early atmosphere of planets (Nat. Comm., 2021). And the isotope effect in the photochemistry of water molecules is an important reason for the uneven distribution of D/H isotopes in the solar nebula (Sci. Adv., 2021).
the related research results are titled "Vibrationally Excited Molecular Hydrogen Production from the Water Photochemistry". It was recently published in Nature Communications. The first author of this work is Chang Yao, a postdoctoral fellow in Group 2507 of Dalian Institute of Chemical Physics. This work was supported by the National Natural Science Foundation of China's Frontier Research Center for Dynamic Chemistry, the B-class Strategic Priority Research Program of the Chinese Academy of Sciences on "The Essence and Regulation of Energy Chemical Conversion", the National Natural Science Foundation of China's Outstanding Youth Program, and the Liaoning Province Xingliao Talent Program, among others. (Written and photographed by Chang Yao and Yuan Kaijun
The article links: https://doi.org/10.1038/s41467-021-26599-9
(The text and images are reprinted from the homepage of Dalian Institute of Chemical Physics, Chinese Academy of Sciences)